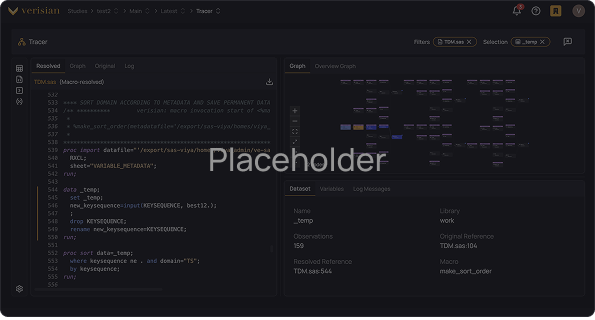
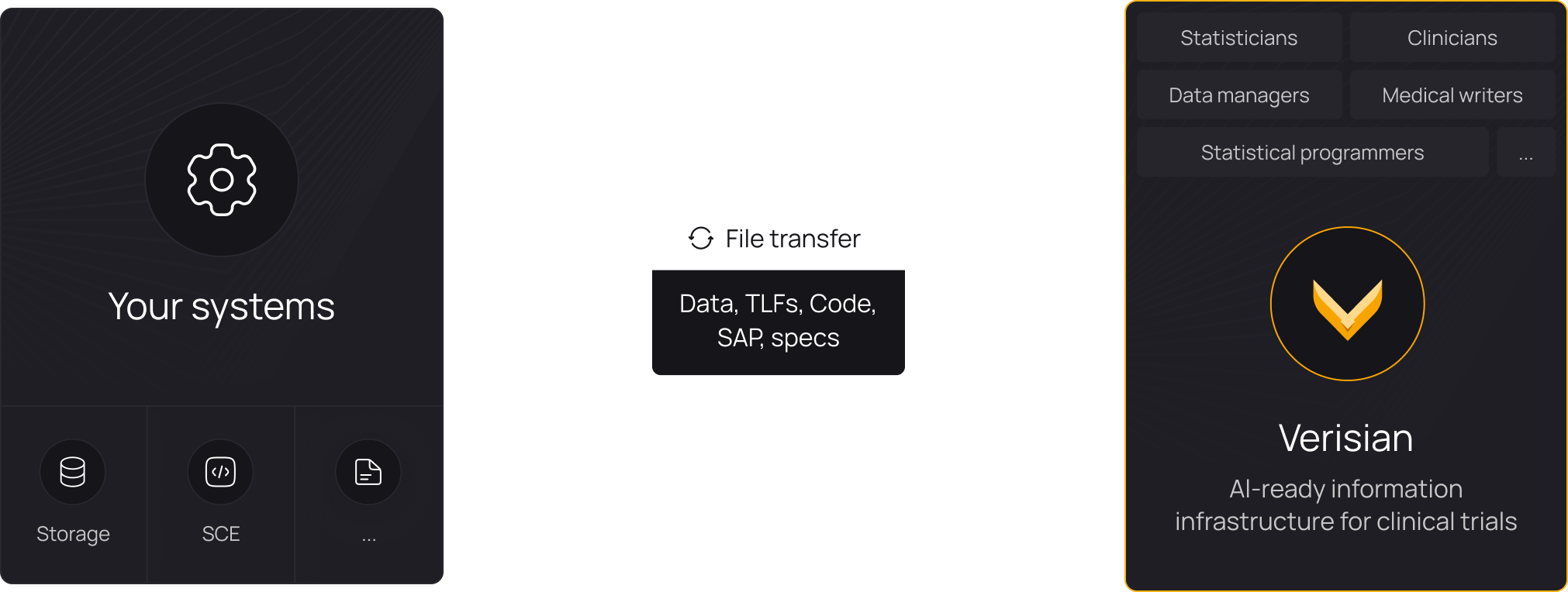
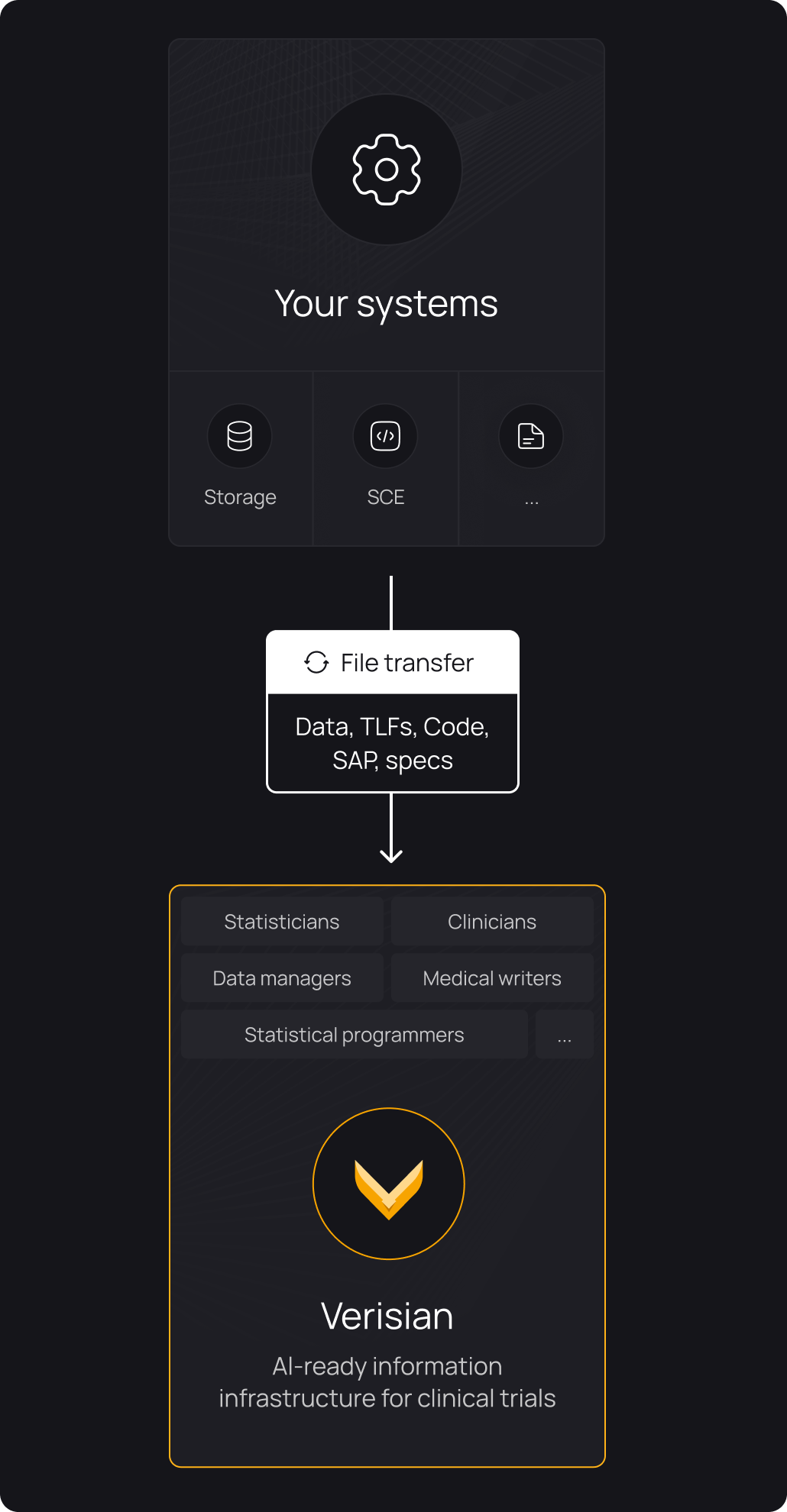
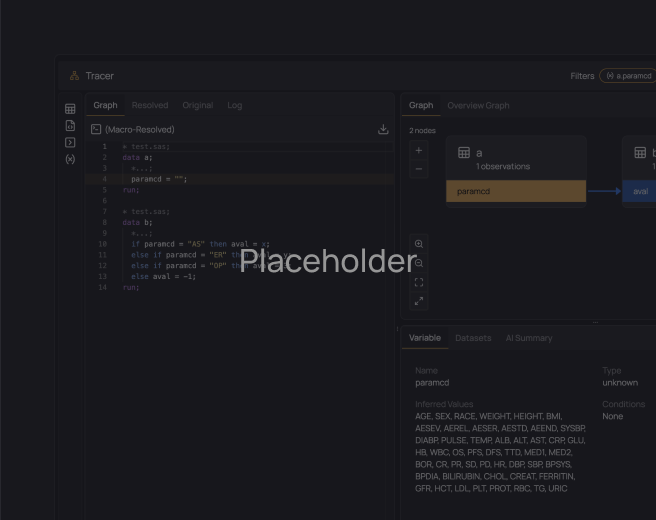
Onboard new programmers to studies 50-80% faster
Give every programmer instant, fully traceable access to variables, derivations, data, TLFs, and their relationships. Any programmer can open a study in Verisian and leverage code traceability to gain a complete understanding of the study